www.magazine-industry-usa.com
07
'25
Written on Modified on
Hydrogen Fuel Cell Vehicles
Hydrogen fuel cells have an important role to play in clean mobility, says Milton D’Silva.
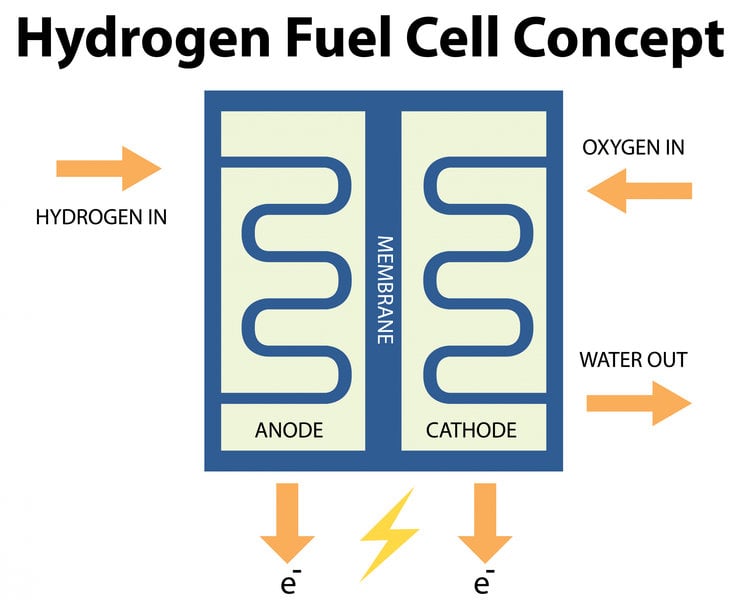
Hydrogen fuel cell concept. Image by brgfx on Freepik
As the most abundant element in the universe and one that has been around from the time of the Big Bang, Hydrogen has an interesting history. British scientist Henry Cavendish identified it as a distinct element in 1766, but the name hydrogen was given by French chemist Antoine Lavoisier in 1773. In 1799, Phillipe Lebon, a French civil engineer with keen interest in the then-evolving steam engines, had mentioned the possibility of using hydrogen, among other gases, for ‘machine movement’. Lebon is credited as the inventor of ‘gaslights’ and had in fact obtained a patent for a gas engine, but was murdered on the streets of Paris in 1804, before he could produce a prototype. There were others working on the same idea and records exist of such attempts, including one by Swiss engineer Francois Isaac de Rivaz who built an internal combustion (IC) engine powered by a mix of hydrogen and oxygen in 1806. The car Rivaz built the following year, however, did not succeed, but it proved the feasibility of a hydrogen-powered automobile. The first functional hydrogen-powered automobile was a three-wheeled contraption called Hippomobile, developed by Belgian inventor Etienne Lenoir in 1860, powered by a single-cylinder two-stroke engine. The hydrogen for this was produced by the process of electrolyzing water.
The first fuel cell was also developed almost 200 years ago, in 1839 by British physicist Sir William Robert Grove. This early fuel cell, called the Grove cell, was made by making use of zinc and platinum electrodes dipped in acid to produce electricity. In 1842, Grove succeeded in producing electrical energy by combining hydrogen and oxygen, but it was not put to any practical use other than in some cases by the early telegraphic industry, without much success. However, it demonstrated the concept of the hydrogen fuel cell for the first time. The term ‘fuel cell’ was coined by two British chemists, Ludwig Mond and Charles Langer, in 1889, when they were working on a fuel cell fed by coal gas. In 1932, English engineer Francis Thomas Bacon successfully developed a 5 kW stationary fuel cell, which was later modified at General Electric and used by NASA for human space flights under Project Gemini.
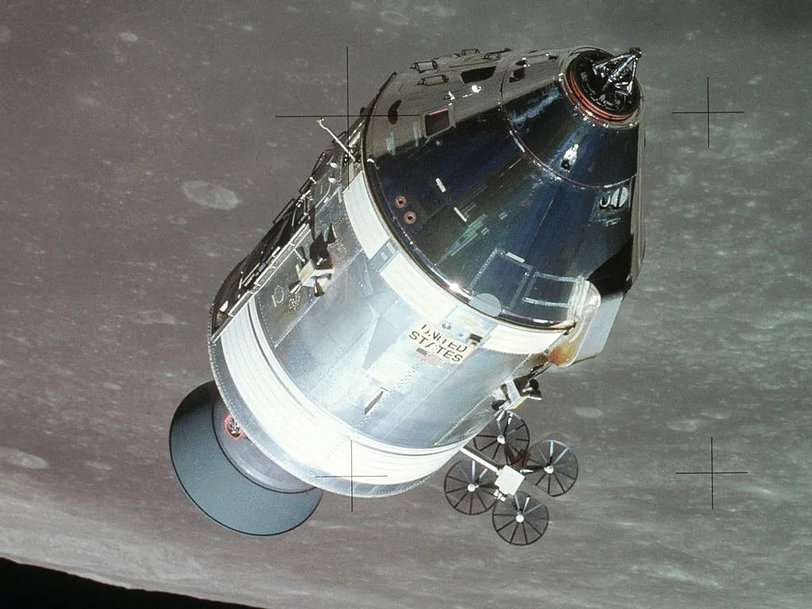
First use of hydrogen fuel cells by NASA in its moon missions. Image source: NASA
The above examples from the past indicate that there was much awareness about the potential of hydrogen as an energy source and efforts were made to harness it. At the same time, both use cases of hydrogen for mobility – as fuel for IC engine and as a fuel cell for generating electricity – were demonstrated albeit in primitive form, technologies that have matured today. Hydrogen burns relatively clean in IC engines, producing primarily water vapour as a byproduct, which means it does not emit carbon dioxide (CO2), making it a cleaner alternative to traditional fossil fuels like gasoline or diesel; however, it can still produce small amounts of nitrogen oxides (NOx) depending on combustion conditions, which can be minimised with suitable engine modifications and/or exhaust treatment systems. When used in fuel cells, hydrogen produces only water and air, making it a clean alternative to ICE vehicles. Thus hydrogen has great potential as a clean alternative to conventional IC engines for mobility, and can play a complementary role to battery electric vehicles (BEV), especially for heavy commercial vehicles, shipping and even aviation, as the world inches forward towards the Net Zero goal.
This article examines at length the use of hydrogen for mobility through the HFCV (Hydrogen Fuel Cell Vehicle) route, where hydrogen, along with oxygen is used as input in the fuel cell to generate electricity, which than is used to run motors that drive the vehicle, or the Fuel Cell Electric Vehicle (FCEV) as it is conventionally known.
Hydrogen Fuel Cell Technology
Hydrogen was first produced by electrolysis in 1800 by English scientists William Nicholson and Sir Anthony Carlisle by using electric current to split water into hydrogen and oxygen. The underlying principle of hydrogen fuel cell technology is the reverse of electrolysis, where hydrogen and oxygen undergo an electrochemical reaction to produce water and electricity. This is an electrochemical process, much like that of conventional lead acid batteries, but with a crucial difference. Unlike batteries, fuel cells do not run down nor need recharging, as long as they are supplied with hydrogen.
What exactly is a fuel cell and how does it work? A typical hydrogen fuel cell is constructed with two electrodes – an anode and a cathode – that are separated by an electrolyte membrane. The membrane is made of solid polymer, which allows protons to pass through but not electrons, facilitating the flow of electrical current. The anode is where hydrogen is introduced, and the cathode is where oxygen is introduced, allowing the reaction to produce electricity, water, and heat as byproducts; both electrodes often contain a catalyst like platinum to facilitate the chemical reaction. The role of platinum, which acts as a catalyst, is important as it not only facilitates the chemical reaction, but also does it much more efficiently than other catalysts. However, platinum is very expensive and adds to the cost; besides it also degrades unevenly as a result of which still usable platinum gets discarded when old and worn-out fuel cells are replaced. There is ongoing research at various quarters to discover other potential catalysts for fuel cells, including iron-nitrogen-carbon compounds, iron-based catalysts, manganese-based catalysts, and alloys like Pt3Ni, which have shown promising results.
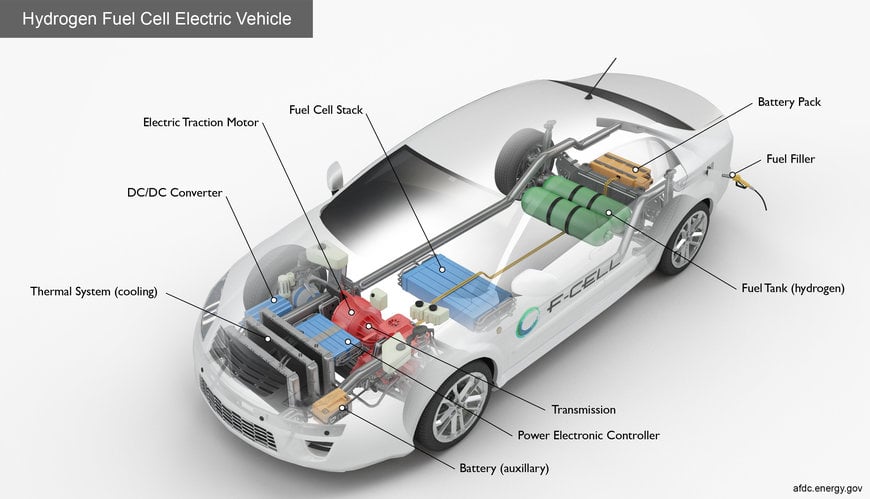
Schematic explaining how fuel cell electric vehicles work. Image source: Alternative Fuels Data Center (US Department of Energy)
There are many different types of fuel cells and these include:
1. Alkaline fuel cells (AFC): These use hydrogen as fuel and are sensitive to carbon dioxide. They are primarily used in controlled aerospace and underwater applications. They were used in the Apollo space program by NASA, and are highly efficient, producing heat and water along with electricity.
2. Phosphoric acid fuel cells (PAFC): They use phosphoric acid as an electrolyte and operate at about 200°C. They are used for stationary power generation in buildings, hotels, hospitals, and utilities.
3. Solid oxide fuel cells (SOFC): These use hydrogen, natural gas, biogas, and other hydrocarbons as fuel, and are considered more efficient than traditional power generation. They operate at high temperatures between 1000-1800 degrees F and use a solid ceramic electrolyte.
4. Proton exchange membrane fuel cells (PEMFC): They use a water-based acidic polymer membrane as the electrolyte and operate at 50-100°C. They are compact, lightweight, and suitable for transport applications.
Molten carbonate fuel cells (MCFC): These are also among the major types of fuel cells, primarily used for large-scale, stationary power generation, mainly due to their ability to efficiently utilise natural gas, coal gas, or biogas as fuel sources while also offering potential for carbon capture from flue gases.
Other than the above five types, there are a few types of fuel cells including:
As the most abundant element in the universe and one that has been around from the time of the Big Bang, Hydrogen has an interesting history. British scientist Henry Cavendish identified it as a distinct element in 1766, but the name hydrogen was given by French chemist Antoine Lavoisier in 1773. In 1799, Phillipe Lebon, a French civil engineer with keen interest in the then-evolving steam engines, had mentioned the possibility of using hydrogen, among other gases, for ‘machine movement’. Lebon is credited as the inventor of ‘gaslights’ and had in fact obtained a patent for a gas engine, but was murdered on the streets of Paris in 1804, before he could produce a prototype. There were others working on the same idea and records exist of such attempts, including one by Swiss engineer Francois Isaac de Rivaz who built an internal combustion (IC) engine powered by a mix of hydrogen and oxygen in 1806. The car Rivaz built the following year, however, did not succeed, but it proved the feasibility of a hydrogen-powered automobile. The first functional hydrogen-powered automobile was a three-wheeled contraption called Hippomobile, developed by Belgian inventor Etienne Lenoir in 1860, powered by a single-cylinder two-stroke engine. The hydrogen for this was produced by the process of electrolyzing water.
The first fuel cell was also developed almost 200 years ago, in 1839 by British physicist Sir William Robert Grove. This early fuel cell, called the Grove cell, was made by making use of zinc and platinum electrodes dipped in acid to produce electricity. In 1842, Grove succeeded in producing electrical energy by combining hydrogen and oxygen, but it was not put to any practical use other than in some cases by the early telegraphic industry, without much success. However, it demonstrated the concept of the hydrogen fuel cell for the first time. The term ‘fuel cell’ was coined by two British chemists, Ludwig Mond and Charles Langer, in 1889, when they were working on a fuel cell fed by coal gas. In 1932, English engineer Francis Thomas Bacon successfully developed a 5 kW stationary fuel cell, which was later modified at General Electric and used by NASA for human space flights under Project Gemini.

First use of hydrogen fuel cells by NASA in its moon missions. Image source: NASA
The above examples from the past indicate that there was much awareness about the potential of hydrogen as an energy source and efforts were made to harness it. At the same time, both use cases of hydrogen for mobility – as fuel for IC engine and as a fuel cell for generating electricity – were demonstrated albeit in primitive form, technologies that have matured today. Hydrogen burns relatively clean in IC engines, producing primarily water vapour as a byproduct, which means it does not emit carbon dioxide (CO2), making it a cleaner alternative to traditional fossil fuels like gasoline or diesel; however, it can still produce small amounts of nitrogen oxides (NOx) depending on combustion conditions, which can be minimised with suitable engine modifications and/or exhaust treatment systems. When used in fuel cells, hydrogen produces only water and air, making it a clean alternative to ICE vehicles. Thus hydrogen has great potential as a clean alternative to conventional IC engines for mobility, and can play a complementary role to battery electric vehicles (BEV), especially for heavy commercial vehicles, shipping and even aviation, as the world inches forward towards the Net Zero goal.
This article examines at length the use of hydrogen for mobility through the HFCV (Hydrogen Fuel Cell Vehicle) route, where hydrogen, along with oxygen is used as input in the fuel cell to generate electricity, which than is used to run motors that drive the vehicle, or the Fuel Cell Electric Vehicle (FCEV) as it is conventionally known.
Hydrogen Fuel Cell Technology
Hydrogen was first produced by electrolysis in 1800 by English scientists William Nicholson and Sir Anthony Carlisle by using electric current to split water into hydrogen and oxygen. The underlying principle of hydrogen fuel cell technology is the reverse of electrolysis, where hydrogen and oxygen undergo an electrochemical reaction to produce water and electricity. This is an electrochemical process, much like that of conventional lead acid batteries, but with a crucial difference. Unlike batteries, fuel cells do not run down nor need recharging, as long as they are supplied with hydrogen.
What exactly is a fuel cell and how does it work? A typical hydrogen fuel cell is constructed with two electrodes – an anode and a cathode – that are separated by an electrolyte membrane. The membrane is made of solid polymer, which allows protons to pass through but not electrons, facilitating the flow of electrical current. The anode is where hydrogen is introduced, and the cathode is where oxygen is introduced, allowing the reaction to produce electricity, water, and heat as byproducts; both electrodes often contain a catalyst like platinum to facilitate the chemical reaction. The role of platinum, which acts as a catalyst, is important as it not only facilitates the chemical reaction, but also does it much more efficiently than other catalysts. However, platinum is very expensive and adds to the cost; besides it also degrades unevenly as a result of which still usable platinum gets discarded when old and worn-out fuel cells are replaced. There is ongoing research at various quarters to discover other potential catalysts for fuel cells, including iron-nitrogen-carbon compounds, iron-based catalysts, manganese-based catalysts, and alloys like Pt3Ni, which have shown promising results.

Schematic explaining how fuel cell electric vehicles work. Image source: Alternative Fuels Data Center (US Department of Energy)
There are many different types of fuel cells and these include:
1. Alkaline fuel cells (AFC): These use hydrogen as fuel and are sensitive to carbon dioxide. They are primarily used in controlled aerospace and underwater applications. They were used in the Apollo space program by NASA, and are highly efficient, producing heat and water along with electricity.
2. Phosphoric acid fuel cells (PAFC): They use phosphoric acid as an electrolyte and operate at about 200°C. They are used for stationary power generation in buildings, hotels, hospitals, and utilities.
3. Solid oxide fuel cells (SOFC): These use hydrogen, natural gas, biogas, and other hydrocarbons as fuel, and are considered more efficient than traditional power generation. They operate at high temperatures between 1000-1800 degrees F and use a solid ceramic electrolyte.
4. Proton exchange membrane fuel cells (PEMFC): They use a water-based acidic polymer membrane as the electrolyte and operate at 50-100°C. They are compact, lightweight, and suitable for transport applications.
Molten carbonate fuel cells (MCFC): These are also among the major types of fuel cells, primarily used for large-scale, stationary power generation, mainly due to their ability to efficiently utilise natural gas, coal gas, or biogas as fuel sources while also offering potential for carbon capture from flue gases.
Other than the above five types, there are a few types of fuel cells including:
- Direct-Methanol Fuel Cells (DMFC) – similar to the PEM cell, but use methanol directly on the anode. These have higher energy density than hydrogen, and are ideal for portable electronic devices, such as laptops and battery rechargers.
- Combined Heat and Power Fuel Cells (CHPFC) – these produce heat in addition to electricity, which is then used for heating, including hot water and space heating, and are ideal for powering houses and buildings.
- Regenerative or Reversible Fuel Cells – this is an emerging trend, a special class of fuel cells that produce electricity from hydrogen and oxygen, but can be reversed and powered with electricity to produce hydrogen and oxygen.
The most common type of fuel cell used in vehicles is the polymer electrolyte membrane (PEM) fuel cell, also called a proton exchange membrane fuel cell, which primarily uses hydrogen as fuel and is considered the best option for automotive applications.
What makes the PEM fuel cell ideal for vehicles? For that one has to look at its characteristics. PEM fuel cells operate at relatively low temperatures, allowing for quick start-up times, have high power density, and can rapidly adjust its output to meet fluctuating power demands, which make them highly suitable for the dynamic needs of driving a car. The fact that these produce minimal emissions, making it environmentally friendly, is a given.
The evolution of fuel cells and automotive applications
With a legacy of over 200 years from concept and early experiments, the fuel cell has come a long way. Between the Grove cell designed in 1839 by Sir William Robert Grove to the 5 kW stationary fuel cell developed by Francis Thomas Bacon in England in 1932, there were several others who had worked on the same idea with different gases and cell materials, without really achieving the desired results. However, the idea of the fuel cell was finally brought to life by Bacon with his Alkaline Fuel Cell. But the credit of putting the fuel cell into practical use goes to NASA, and in particular to the Project Gemini of the early 1960s and later, the Apollo Moon Mission. Until then, fuel cells were just a concept that had never been put to practical use. At a time when the world was still using fossil fuels, engineers at NASA relied on fuel cells because they could provide more energy per unit weight than batteries over the course of a long mission. For that, they chose Bacon's design of the Alkaline Fuel Cell, which consumed hydrogen and pure oxygen, to produce potable water, heat, and electricity. The Bacon cell was further refined first by engineers at GE and later at Pratt & Whitney, the company that played a key role in developing fuel cells for NASA's Apollo and Space Shuttle programs. The Pratt & Whitney group company that developed the fuel cells later became UTC Power, and a supplier of fuel cells to all the space shuttles. In 2014, UTC Power was acquired by Doosan Group, an energy-focused multinational industrial powerhouse from Korea, and renamed HyAxiom, promoting the adoption of hydrogen-based energy solutions worldwide.
What makes the PEM fuel cell ideal for vehicles? For that one has to look at its characteristics. PEM fuel cells operate at relatively low temperatures, allowing for quick start-up times, have high power density, and can rapidly adjust its output to meet fluctuating power demands, which make them highly suitable for the dynamic needs of driving a car. The fact that these produce minimal emissions, making it environmentally friendly, is a given.
The evolution of fuel cells and automotive applications
With a legacy of over 200 years from concept and early experiments, the fuel cell has come a long way. Between the Grove cell designed in 1839 by Sir William Robert Grove to the 5 kW stationary fuel cell developed by Francis Thomas Bacon in England in 1932, there were several others who had worked on the same idea with different gases and cell materials, without really achieving the desired results. However, the idea of the fuel cell was finally brought to life by Bacon with his Alkaline Fuel Cell. But the credit of putting the fuel cell into practical use goes to NASA, and in particular to the Project Gemini of the early 1960s and later, the Apollo Moon Mission. Until then, fuel cells were just a concept that had never been put to practical use. At a time when the world was still using fossil fuels, engineers at NASA relied on fuel cells because they could provide more energy per unit weight than batteries over the course of a long mission. For that, they chose Bacon's design of the Alkaline Fuel Cell, which consumed hydrogen and pure oxygen, to produce potable water, heat, and electricity. The Bacon cell was further refined first by engineers at GE and later at Pratt & Whitney, the company that played a key role in developing fuel cells for NASA's Apollo and Space Shuttle programs. The Pratt & Whitney group company that developed the fuel cells later became UTC Power, and a supplier of fuel cells to all the space shuttles. In 2014, UTC Power was acquired by Doosan Group, an energy-focused multinational industrial powerhouse from Korea, and renamed HyAxiom, promoting the adoption of hydrogen-based energy solutions worldwide.
The success of the fuel cells in space programmes inspired a whole lot of researchers in adapting the technology for use on earth and the obvious choice was vehicles. In 1966, General Motors became the first company to power a passenger vehicle with a fuel cell when it converted an existing model, the Handivan, into the Chevrolet Electrovan. Only one vehicle was thus converted, powered by a fuel cell that combined liquid oxygen and supercooled liquid hydrogen. The Electrovan achieved a top speed of 70 mph, with a range of about 150 miles, could accelerate from 0-60 MPH in 30 seconds, and carry two passengers as most of the space was taken up by the equipment. It was deemed too expensive and not safe enough for roads so it was run only within the plant, but proved a point. The fuel cell for passenger cars was no longer a concept. The Electrovan paved the way for the modern HFCVs that followed in due course.
Several developments in the 1970s led to a revival of interest in the automobile industry for the hydrogen fuel cell technology as a possible option. As NASA’s Apollo mission came to an end, a large number of professionals with expertise in fuel cells were available to work in the automobile industry that was also experiencing the aftermath of the oil crises then plaguing the world, most notably the oil shocks of 1973 and 1979 and the subsequent OPEC embargoes. This prompted automakers and governments to explore alternative fuels to reduce dependence on oil. At the same time there was rising awareness of air pollution and climate change, which led to stricter emission regulations. Hydrogen, with its near-zero emissions in fuel cells and even when used as fuel with IC engines, offered itself as an attractive option. This was also accompanied by continuous improvements in hydrogen storage, fuel injection, and engine design that made HFCVs more viable as research in hydrogen internal combustion engines continued alongside hydrogen fuel cells. In fact major automakers like BMW, Mazda, Ford, Honda, Toyota and Hyundai, had already started experimenting with hydrogen combustion engines in the 1980s and 1990s, or had plans to do so.
BMW has a long history of researching hydrogen as a fuel for IC engines in its vehicles, and had demonstrated the first vehicle powered by hydrogen as a fuel as early as in 1979. In early September 2024, BMW and Toyota signed an agreement to strengthen their collaboration in the hydrogen sector, which the two companies had first signed in 2011. A statement released on the occasion mentioned them sharing a common vision of ‘realizing a hydrogen society’ to accelerate technological innovation in fuel cell systems. To that end, the two companies are jointly developing a third-generation fuel cell system and working on infrastructure development co-creation. As a first step, BMW plans to launch its first mass-produced FCEV in 2028.
Mazda of Japan has also researched the use of hydrogen as a fuel for IC engines for a long time and developed hydrogen rotary engines and fuel cell electric vehicles. The company has in fact developed a hydrogen rotary engine that combines port fuel injection (PFI) and direct injection (DI) technology and integrated it in a sports car model.
For Ford Motor Company, one of the original Big 3 of Detroit, research into hydrogen vehicles began in the late 1990s with the P2000 hydrogen engine design and development programme. The idea was to develop the hydrogen infrastructure before the widespread use of fuel cell vehicles. Though the project was later dropped due to poor fuel efficiency, it generated a wealth of data. Today the company is primarily researching hydrogen fuel cell technology for heavy-duty vehicles like trucks and vans, rather than passenger cars, and has partnered with Ballard Power Systems to develop a hydrogen fuel cell system for their trucks. Currently, it is also conducting trials with hydrogen fuel cell powered E-Transit vans in the UK, exploring the viability of this technology in real-world scenarios.
Presently, commercially available FCEVs in production are the Toyota Mirai and Hyundai Nexo. However, these models are primarily available in regions with established hydrogen refueling infrastructure, often concentrated in California in the US or in certain regions of Japan and Korea. Recently, Honda has also started producing a limited quantity of the Honda CR-V e:FCEV. This model features a plug-in charging function that enables charging of the onboard battery from an external power source as well, which further enhances the convenience of the hydrogen fuel cell powered vehicle.
Apart from its collaboration with BMW, Toyota is also working with fellow Japanese automakers Mazda and Subaru on development of new engines and electric drivetrains. The idea is not to just rely on battery electric vehicles (BEV) for decarbonisation, but work towards carbon neutrality with a range of emission free engine options. Identifying carbon as the enemy, the three companies will be working on developing new engines which will be made carbon neutral by shifting away from fossil fuels and offering compatibility with various alternatives, including e-fuel (synthetic fuel), biofuels, and liquid hydrogen. CEOs of the three companies appeared on a single platform, the Multipathway Workshop in May 2024. Speaking on the occasion, Koji Sato, President, Member of the Board of Directors and CEO, Toyota Motor Corporation, said, “In order to provide our customers with diverse options to achieve carbon neutrality, it is necessary to take on the challenge of evolving engines that are in tune with the energy environment of the future. The three companies, which share the same aspirations, will refine engine technologies through friendly competition.”

A Toyota hydrogen fuel cell concept vehicle on display in Tokyo, 2019. Photo by Darren Halstead on Unsplash
Advantages of hydrogen fuel cell vehicles
When it comes to the contemporary world energy scenario, the transport sector with its heavy dependence on fossil fuels accounts for over a third of global CO2 emissions. At the moment, the battery electric vehicle (BEV) market, which has had a head start and support from various government initiatives and subsidies, has grown impressively. Yet there are significant roadblocks ahead, in terms of clean electricity to fuel the growing charging infrastructure as well as the electrification of heavy commercial and public transport fleets where the batteries are inadequate to support long travel range. It is this gap that hydrogen vehicles can bridge, which makes the fuel cell an important part of the solution.
Any new technology that comes to the market is initially overpriced due to a host of factors and so it was in the case of EV batteries. Now thanks to growing adoption, mass production and newer technologies, battery prices have come down to more realistic levels. According to one estimate, prices of lithium-ion battery packs decreased by 89% between 2010 and 2020. Similar is the trend in case of hydrogen fuel cells where prices are showing a declining trend due to several factors. These include: manufacturing improvements, increased competition among producers, government incentives, and economies of scale as more fuel cells are produced, leading to more efficient production processes and lower overall costs per unit. Along with the decreasing cost of hydrogen production and scaling up of refuelling infrastructure, over the next few years there will be a significant boost to the hydrogen economy – the vision for a future energy system that uses hydrogen as a clean fuel to reduce carbon emissions.

Koji Sato, President of Toyota Motor Corporation, and Oliver Zipse, Chairman of the Board of Management of BMW AG. Photo: Toyota Press
Reduced carbon emissions is the major advantage of FCEVs, but it is not the only one. The other benefits include:
- Zero tailpipe emissions: FCEVs produce only water vapour and heat, which means they don't emit harmful greenhouse gases or other pollutants.
- Fuel economy: FCEVs can get about twice the mileage of gasoline vehicles.
- Renewable energy: Hydrogen can be produced from renewable energy sources, clean electricity and water, through the electrolysis process.
- Energy diversity: FCEVs can help diversify the energy sources used in a country, which can help reduce reliance on fossil fuels.
- Longer driving range: FCEVs can have a driving range of up to 500 kilometres, comparable to conventional cars.
- Quick refueling: FCEVs can be refueled in about 5 minutes, which is about the time it takes to refuel a regular car.
- Reliable: The quality of power provided by hydrogen fuel cells doesn't degrade over time.
- Lightweight: Hydrogen fuel cells have a high energy to weight ratio, so they're lighter and take up less space than lithium-ion batteries.
The flip side
Not everything is perfect and hunky-dory on hydrogen as an alternative fuel in general, hydrogen fuel cells in particular. FCEVs also have some distinct disadvantages, including:
Not everything is perfect and hunky-dory on hydrogen as an alternative fuel in general, hydrogen fuel cells in particular. FCEVs also have some distinct disadvantages, including:
- Cost: FCEVs are expensive, and the fuel cells can be expensive to replace
- Infrastructure: There is limited infrastructure for FCEVs, such as hydrogen fueling stations
- Safety: There are safety concerns about hydrogen's flammability and electrical shock
- Transportation: Hydrogen is difficult to transport and handle
As for the cost, as and when adoption grows especially with new generation models, the prices would show a downward trend, as has happened in case of BEVs. In the short term future, BEVs will certainly dominate the clean mobility market in the passenger segment, but FCEVs will have a greater acceptability in the commercial vehicles and mass transport sector, also in marine and aviation industries. Already several cities are using hydrogen fuel cell buses for public transit, offering clean emissions and reduced noise pollution while maintaining long operational hours. Similarly, hydrogen fuel cells are used in warehouses and industrial settings to power forklifts and other material handling equipment due to their ability to operate continuously without needing frequent battery recharging. With adequate support from the government and the fossil fuel industry now under pressure to clean up its act, the infrastructure too is expected to scale up rapidly, so is the case with stringent safety measures with evolvings standards.
The most important factor however is not the fuel cell per se, but the source of hydrogen. The cheapest form of hydrogen today is ‘Grey’ hydrogen, which is produced from natural gas, or methane, using the Steam Methane Reforming (SMR) method. During this process, carbon dioxide (CO2) is produced as a byproduct, and greenhouse gases released to the atmosphere. This is clearly not an environmentally friendly alternative. An improvement over the ‘Grey’ type, ‘Blue’ hydrogen is produced with the same SMR method using natural gas, similar to grey hydrogen, but there is an additional step to reduce carbon emissions. The additional step is the process of carbon capture and storage (CCS), a technology used to capture the CO2 emissions generated during the process. This makes Blue hydrogen expensive compared to Grey. For fuel cells to be considered truly emission free, the hydrogen used must be ‘Green’, produced through the electrolysis process using electricity. What sets green hydrogen apart is that the electricity used in the process comes from renewable energy sources, such as wind, solar, or hydropower, and not generated with fossil fuels, making it the most appropriate fuel for a fully sustainable energy transition.

Atsushi Osaki, President and CEO, Subaru Corporation; Koji Sato, President, Toyota Motor Corporation; and Masahiro Moro, President and CEO, MAZDA Motor Corporation. Photo: Toyota Press
Conclusion
As the world is exploring ways to reach the goal of Net Zero by 2050 in line with the understanding implicit in the Paris Agreement, hydrogen has an important role to play in this transition. Hydrogen can ably complement other decarbonisation technologies like renewable power, biofuels, or energy efficiency improvements. Besides applications for clean mobility, hydrogen fuel cells are also used for backup power generation as stationary hydrogen fuel cell systems are implemented in critical infrastructure like hospitals and data centres as a reliable backup power source in case of grid outages. In addition, hydrogen fuel cells can be utilised in remote areas with limited access to the electricity grid to provide power for homes, communication towers, and other essential services.
According to the International Renewable Energy Agency (IRENA), a lead global intergovernmental agency for energy transformation, in 2017 just one country – Japan – had a national hydrogen strategy. Today, more than 30 countries have developed or are preparing hydrogen strategies, indicating growing interest in developing hydrogen value chains, it notes. This augurs well for a world in quest of the Net Zero goal.
References
The most important factor however is not the fuel cell per se, but the source of hydrogen. The cheapest form of hydrogen today is ‘Grey’ hydrogen, which is produced from natural gas, or methane, using the Steam Methane Reforming (SMR) method. During this process, carbon dioxide (CO2) is produced as a byproduct, and greenhouse gases released to the atmosphere. This is clearly not an environmentally friendly alternative. An improvement over the ‘Grey’ type, ‘Blue’ hydrogen is produced with the same SMR method using natural gas, similar to grey hydrogen, but there is an additional step to reduce carbon emissions. The additional step is the process of carbon capture and storage (CCS), a technology used to capture the CO2 emissions generated during the process. This makes Blue hydrogen expensive compared to Grey. For fuel cells to be considered truly emission free, the hydrogen used must be ‘Green’, produced through the electrolysis process using electricity. What sets green hydrogen apart is that the electricity used in the process comes from renewable energy sources, such as wind, solar, or hydropower, and not generated with fossil fuels, making it the most appropriate fuel for a fully sustainable energy transition.

Atsushi Osaki, President and CEO, Subaru Corporation; Koji Sato, President, Toyota Motor Corporation; and Masahiro Moro, President and CEO, MAZDA Motor Corporation. Photo: Toyota Press
Conclusion
As the world is exploring ways to reach the goal of Net Zero by 2050 in line with the understanding implicit in the Paris Agreement, hydrogen has an important role to play in this transition. Hydrogen can ably complement other decarbonisation technologies like renewable power, biofuels, or energy efficiency improvements. Besides applications for clean mobility, hydrogen fuel cells are also used for backup power generation as stationary hydrogen fuel cell systems are implemented in critical infrastructure like hospitals and data centres as a reliable backup power source in case of grid outages. In addition, hydrogen fuel cells can be utilised in remote areas with limited access to the electricity grid to provide power for homes, communication towers, and other essential services.
According to the International Renewable Energy Agency (IRENA), a lead global intergovernmental agency for energy transformation, in 2017 just one country – Japan – had a national hydrogen strategy. Today, more than 30 countries have developed or are preparing hydrogen strategies, indicating growing interest in developing hydrogen value chains, it notes. This augurs well for a world in quest of the Net Zero goal.
References
- https://afdc.energy.gov/vehicles/how-do-fuel-cell-electric-cars-work
- https://www.nasa.gov/missions/apollo/tech-today-nasas-moonshot-launched-commercial-fuel-cell-industry/
- https://www.irena.org/Energy-Transition/Policy/Policies-for-green-hydrogen

