www.magazine-industry-usa.com
23
'13
Written on Modified on
CIP
De Dietrich offers cutting-edge CIP (Clean In Place) Systems for Pharmaceutical and Chemical companies.
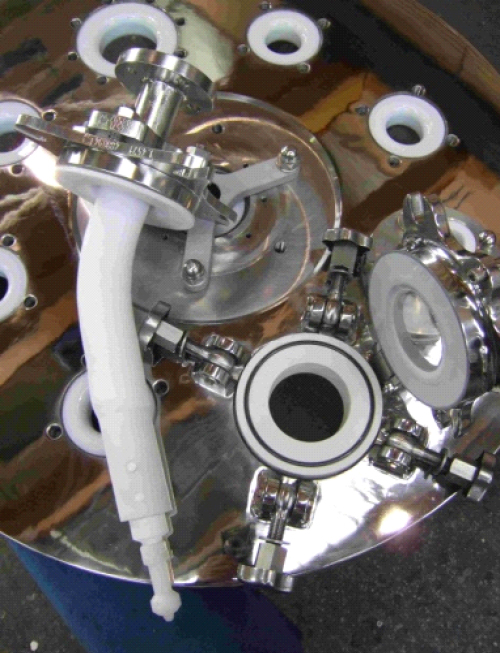
Analytical approach
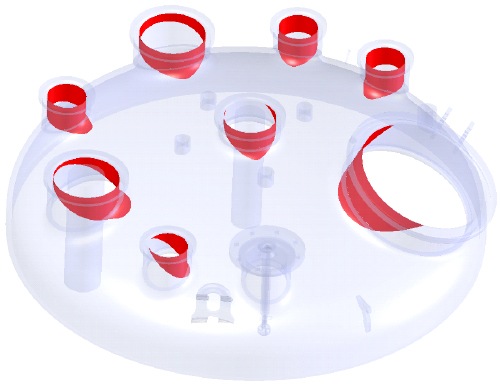
The success of CIP technology is based on the coverage of the solvent and the internal equipment parts. The main difficulty for project managers or engineers is to ensure that the CIP system will have full coverage. De Dietrich has developed a specific approach and a high- quality process that solves the full coverage problem. With this new method, CIP component selection and optimizing is very easy. A specific numeric simulation is performed on the equipment to obtain the requested coverage, and the report is precise on type, number and localization of each spray-ball. This high-value simulation can be done for existing equipment or for equipment in the process of being manufactured.

On-Site Testing
To validate equipment performances, Riboflavine or Conductivity tests can be done on existing equipment in our facilities or on customer site. With this additional step, De Dietrich experts demonstrate that requested coverage is met.
The expansion of the De Dietrich process provides a guarantee for pharmaceutical and chemical companies that the cleanability of equipment will be optimized and conform to their needs. Furthermore, all requested information (flow rate, pressure) is known in the early stages of the project.
De Dietrich offers numerous advantages for clients, from cleanability studies and tests to optimization of tank and unit designs. The company offers an extensive range of high-efficiency CIP equipment, and provides complete engineering solutions for fixed and movable CIP skids and units.
GMP regulations (part 133,4) and similar (part 211.67) starts by: Equipment....shall be maintained in a clean and orderly manner....
The main concerns of the FDA concerning cleanability are:
Avoiding contamination of product
Avoiding cross contamination (by improper cleaning, bad maintenance)
“Call back” action of product.

